Battery Design Blog Posts
Why Car Batteries Perform Poorly in Cold Weather
Have you ever gone to start your car on a cold winter morning and nothing happens? The battery’s ability to convert chemical energy into electrical energy can be affected by low temperatures.
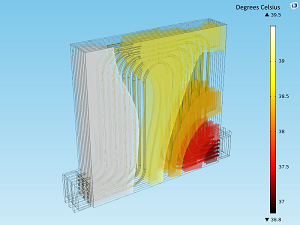
Accounting for Heat in the Design of Lithium-Ion Batteries
Heat transfer is an important phenomenon to consider when designing a lithium-ion battery. See how multiphysics simulation can help ensure a safe and efficient Li-ion battery design.
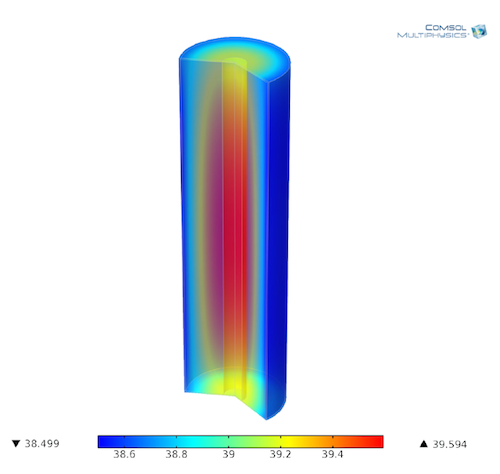
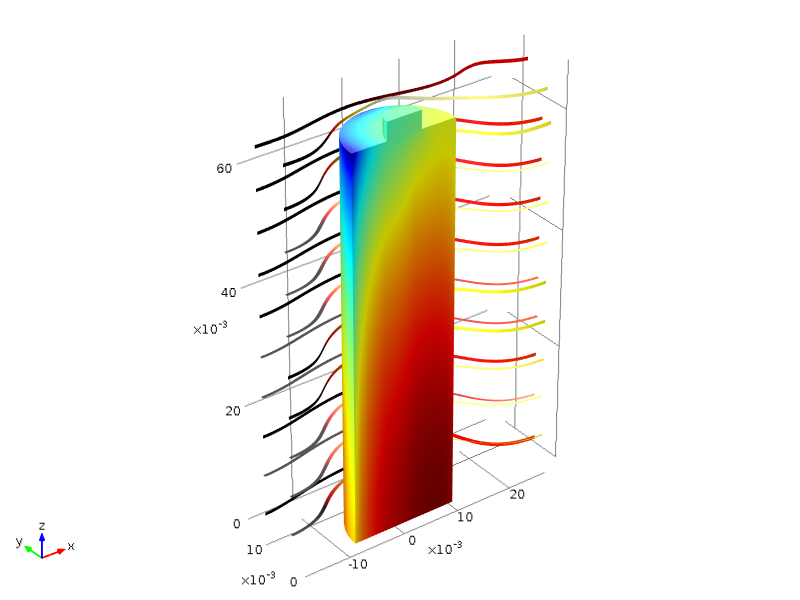
Modeling Lithium-Ion Batteries for Quality and Safety Assurance
Intertek Semko AB assesses more than 20,000 lithium-ion batteries per year. Learn about how they use COMSOL Multiphysics® to understand and analyze their battery and fuel cell designs.
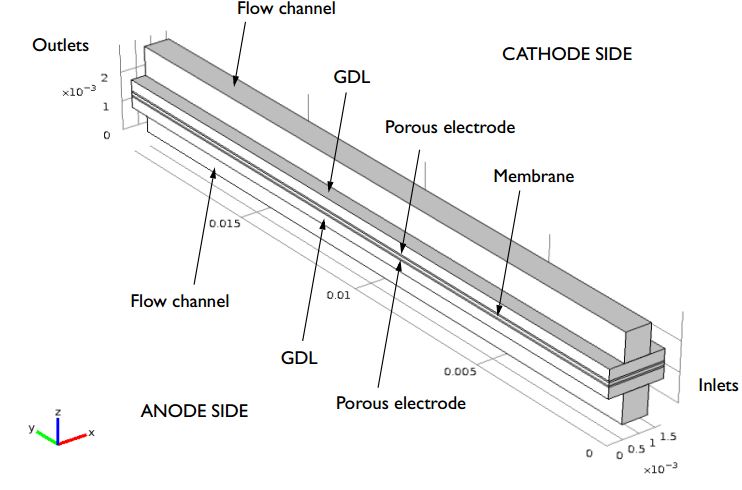
PEM Fuel Cell Modeling Examples
What can you study in a proton exchange membrane fuel cell? Mass transport, ohmic losses, temperature distribution, species transport, serpentine flow…should we keep going?
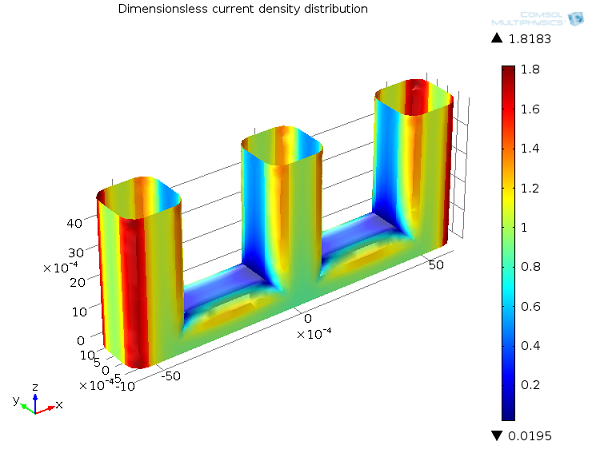
Which Current Distribution Interface Do I Use?
See the different current distributions with a wire electrode example to help you choose between the current distribution interfaces in COMSOL Multiphysics® for your electrochemical simulations.
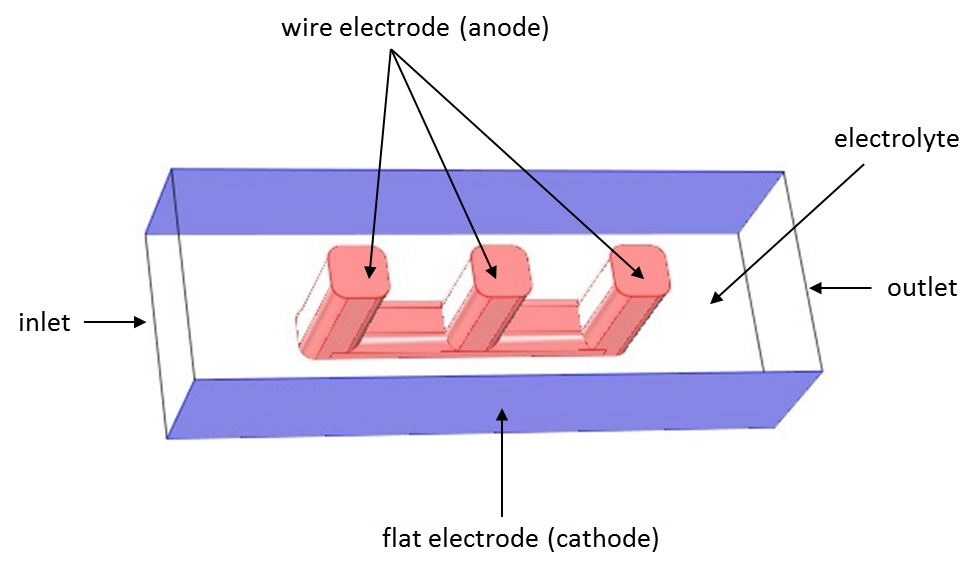
Theory of Current Distribution
Here, we discuss one of the building blocks that make up hybrid parallel computing, namely shared memory computing, as well as when and how to use shared memory with COMSOL Multiphysics®.
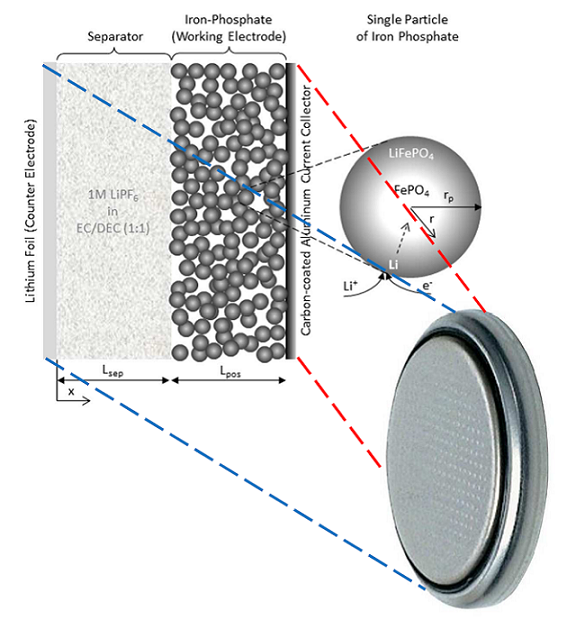
A Lithium-ion Battery Analysis at INES-CEA
During my time as a PhD student, a blue “Chemical Landmark” plaque was fitted to the building a couple of hundred yards down the road from my lab. The plaque commemorates the achievements of the researchers who made the lithium-ion (Li-ion) battery viable. Whether or not you know about the electrochemistry of rechargeable lithium-ion batteries, you probably rely on one. We carry them around in our phones and laptops, and ride in cars and planes that use them for power. […]
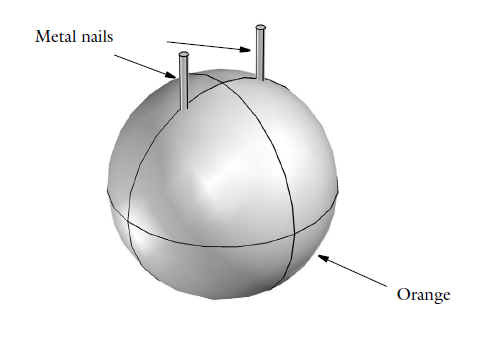
Learn How to Model Electrochemistry with an Orange Battery Tutorial
Did your chemistry teacher use an orange or lemon to demonstrate the concept of a battery, back in the day? You might remember how she magically produced electricity by sticking a couple of metal nails into the citrus fruit, as the whole class watched in awe. What if we now used simulation tools to demonstrate how an orange battery works, and then use that as an intro to electrochemistry modeling?
