Improving Natural Convection in Redox Flow Batteries
Harvesting of renewable energy requires efficient energy storage systems. Rechargeable flow batteries offer certain advantages over other energy storage techniques in use, such as the lifting of water, compression of air, flywheel, etc. In a flow battery, electrolyte flowing through/between the two electrodes, electrical energy is stored as chemical energy through reversible redox electrochemical reactions. Typical applications of large-scale storage include load-leveling, peak shaving, backup power, electric vehicles, etc. While some flow cells have been scaled up, we still need to develop flow batteries with high energy and power densities, large cycle life, and low cost. Soluble lead redox flow battery (SLRFB) is among the least expensive in its class, because of the raw material used and the single electrolyte flow loop which eliminates the expensive proton exchange membrane. Challenges such as limited cycle life and low energy efficiency need to be overcome, however, to take it to the next level. We have earlier established through CFD modeling, measurements, and flow visualization the dominant role of natural convection in SLRFB.
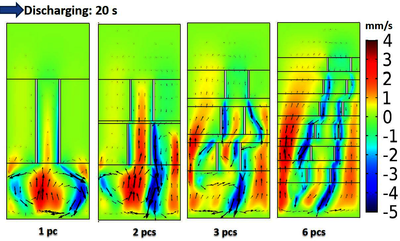
In this work, we present our findings relating to the harnessing of natural convection to minimize external mixing requirements. COMSOL Multiphysics® was used to solve equations for unsteady state two-dimensional fluid flow, species transport, electrochemical reactions, and electro-deposition and electro-dissolution of active material on and from the electrodes. Figure 1a and 1b schematically show a standard and internal lift cell (electrodes lifted off the cell walls) respectively. We have earlier shown left cell to perform better. Figure 1 shows variation of cell potential (Fig. 1c) and the average concentration of Pb(II) on cathode (Fig. 1d) with time during charging for full-length electrodes and the same electrodes split into six equal parts with some horizontal and vertical separation between the segments, while all other parameters the same. The figure shows that the segmented electrodes provide longer charging time compared to the full-length electrode. The depletion of active material in the electrolyte is larger for full-length electrode than the segmented electrode. These findings have inspired more effective variations of electrode configurations to harness natural convection better.
Download
- ansari_presentation.pdf - 0.94MB
- ansari_abstract.pdf - 0.09MB
