Optimizing electrochemical conversion of CO2 to C2 products via multi-physics simulations
The low-temperature electrochemical conversion of CO₂ to ethylene offers significant potential for reducing the chemical industry’s reliance on fossil fuels. Despite recent advancements, the key performance indicators—activity, selectivity, and durability—are insufficient for industrial implementation. The membrane-electrode-assembly (MEA) cell design is promising for achieving higher current densities but introduces challenges such as gas diffusion electrode (GDE) flooding. Recent experiments show that selectivity can be enhanced by using tandem catalysts (silver and copper) to promote in-situ reactions (CO₂ to CO and then CO to C₂H₄) [1]. To optimize the electrochemical cell and operating conditions, a multi-physics computational model is developed at the cell-level to understand the impact of different geometrical features, material properties, and operating variables. Initially, a steady-state isothermal 1D model based on existing literature [2] is developed, assuming a liquid-free gas diffusion layer (GDL). Gas and liquid flow, transport of 14 species (multi-species diffusion), and 13 reactions (heterogeneous and homogeneous) are modeled in the different (porous or open channel) regions. This model is then extended to 2D to capture spatial variations and study the impact of material properties and operating conditions on performance. Different parameter variations have been investigated, including gas feed composition, catalyst loading, catalyst active area, GDL porosity. The impact of these parameters on I-V curve, Faradaic efficiency, and spatial distributions of the key quantities (concentrations) will be reported, and optimal parameter combinations will be shown.
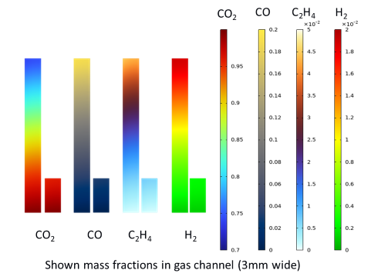
Download
- rajora_9601_poster.pdf - 0.59MB
- 3_aviral_rajora.pdf - 2.25MB
